Answers
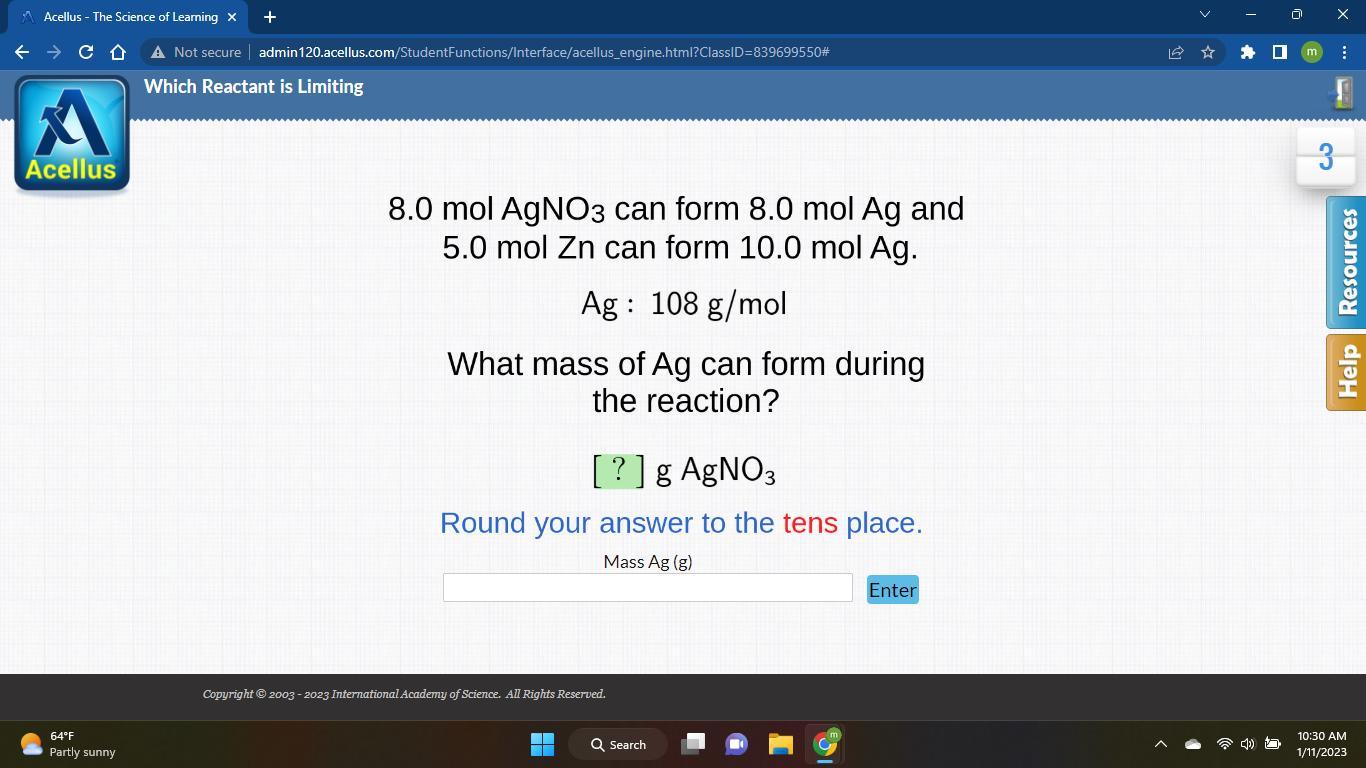
The mass of the Ag formed is 1080 g.
What is the mass formed?Since we do not have the equation of the reaction, it means that we have to write down the equation of the reaction by ourselves so that we can be able to answer the question and we have;
[tex]2AgNO_{3} + Zn ----- > Zn(NO_{3} )_{2} + 2Ag[/tex]
We have that zinc is the limiting reactant so;
If 1 mole of zinc forms 2 moles of Ag
5 moles of zinc forms 5 * 2/1 = 10 moles of Ag
Mass of the Ag = Number of moles * molar mass
= 10 moles * 108 g/mol
= 1080 g
The mass of the silver is 1080 g
Learn more about stoichiometry:https://brainly.com/question/9743981
#SPJ1
Related Questions
write the most efficient reaction to make the esters
Answers
To synthesize esters efficiently, you can use the Fischer esterification reaction. It involves the reaction of a carboxylic acid with an alcohol in the presence of an acid catalyst, usually concentrated sulfuric acid.
The equilibrium can be shifted in favor of ester formation by using an excess of alcohol or removing the water produced during the reaction. Making esters involves a chemical reaction between a carboxylic acid and an alcohol, which can be catalyzed by an acid catalyst. However, there are many different methods and conditions that can be used to make esters depending on the specific carboxylic acid and alcohol involved. The reaction proceeds with the formation of an ester and water as the byproducts.
To know more about esterification visit :-
https://brainly.com/question/16251521
#SPJ11
how many grams of cuso4 · 5h2o are needed to prepare 20 ml solution of concentration 0.5m?
Answers
2.50 grams of [tex]CuSO_4 . 5H_2O[/tex] are needed to prepare a 20 ml solution of 0.5 M concentration.
We first need to determine the molar mass [tex]CuSO_4 . 5H_2O[/tex], which is 249.68 g/mol.
Next, we can use the formula for molarity:
Molarity = moles of solute/volume of solution in liters
To find the number of moles of [tex]CuSO_4 . 5H_2O[/tex] needed for a 20 ml solution of 0.5 M concentration, we can rearrange the formula:
moles of solute = Molarity x volume of solution in liters
moles of solute = 0.5 M x 0.02 L = 0.01 moles
We can use the molar mass to calculate the mass of [tex]CuSO_4 . 5H_2O[/tex] needed:
mass = 0.01 mol x 249.68 g/mol = 2.50 g
To know more about Molarity, here
brainly.com/question/8732513
#SPJ4
identify the three glycolytic enzymes, in order of their pathway sequence, that catalyze irreversible reactions and are bypassed in gluconeogenesis
Answers
The three glycolytic enzymes that are bypassed in gluconeogenesis are hexokinase, phosphofructokinase, and pyruvate kinase. Their bypass allows for the synthesis of glucose from non-carbohydrate precursors.
Glycolysis is the metabolic pathway that converts glucose into pyruvate, which can then enter the citric acid cycle or be converted to lactate or ethanol in certain organisms. It involves a series of ten enzymatic reactions, with the first five being reversible and the last five being irreversible.
The three glycolytic enzymes that catalyze irreversible reactions and are bypassed in gluconeogenesis are:
Hexokinase: This enzyme catalyzes the conversion of glucose to glucose-6-phosphate, which is the first step in glycolysis. It is bypassed in gluconeogenesis by the enzyme glucose-6-phosphatase, which converts glucose-6-phosphate back to glucose.
Phosphofructokinase: This enzyme catalyzes the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate, which is a key regulatory step in glycolysis. It is bypassed in gluconeogenesis by the enzyme fructose-1,6-bisphosphatase, which converts fructose-1,6-bisphosphate back to fructose-6-phosphate.
Pyruvate kinase: This enzyme catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate, the final step in glycolysis. It is bypassed in gluconeogenesis by the enzyme pyruvate carboxylase, which converts pyruvate to oxaloacetate, which can then be converted to phosphoenolpyruvate by the enzyme phosphoenolpyruvate carboxykinase.
To learn more about glycolytic enzymes
https://brainly.com/question/28496909
#SPJ4
Give the structure of the major and minor organic products formed when HBr reacts with (E)-4,4-dimethyl-2-pentene in the presence of peroxides. When drawing hydrogen atoms on a carbon atom, either include all hydrogen atoms or none on that carbon atom, or your structure may be marked incorrect.In each reaction box, place the best reagent and conditions from the list below.
Answers
The structure of the major and minor organic products formed when HBr reacts with (E)-4,4-dimethyl-2-pentene in the presence of peroxides is shown in the image attached.
Reaction of (E)-4,4-dimethyl-2-pentene with HBr by free radical mechanismThe reaction is initiated by the hom---olytic cleavage of H-Br bond to form two free radicals, hydrogen (H•) and bromine (Br•), which are highly reactive and unstable.
The free radical bromine (Br•) reacts with the alkene (E)-4,4-dimethyl-2-pentene to form a more stable carbon-centered free radical intermediate.
The product is washed with aqueous HCl to remove any remaining impurities and neutralize the solution.
Learn more about free radical mechanism:https://brainly.com/question/11631123
#SPJ1
determine the signs of δh°, δs°, and δg° for the following reaction at 125 °c: h2o(g) ⇄ h2o(ℓ) δh° δs° δg°
Answers
The signs of δh°, δs°, and δg° for the reaction H₂O(g) ⇄ H₂O(ℓ) at 125 °C are -ve, -ve, and +ve, respectively.
The sign of δh° depends on whether the reaction is exothermic or endothermic. The transition from gaseous water to liquid water involves the release of heat, indicating an exothermic reaction. Therefore, the sign of δh° will be negative.
The sign of δs° depends on the change in entropy of the system. The randomness of gaseous molecules is greater than that of liquid molecules; thus, the transition from gaseous water to liquid water involves a decrease in entropy. This indicates a negative sign for δs°.
The sign of δg° depends on the spontaneity of the reaction. A negative δg° indicates that the reaction is spontaneous, while a positive δg° indicates that the reaction is non-spontaneous. At a temperature of 125 °C, the boiling point of water, the reaction will proceed in the direction of the gaseous water, which means the reaction is non-spontaneous in the direction of liquid water. Thus, δg° will be positive.
Therefore, the signs of δh°, δs°, and δg° for the reaction H₂O(g) ⇄ H₂O(ℓ) at 125 °C are -ve, -ve, and +ve, respectively.
To know more about exothermic refer here:
https://brainly.com/question/31214950#
#SPJ11
the following tertiary alkyl halide was heated in ethanol for several days, and the resulting mixture of products contained five different elimination products and two substitution products: a)Draw the substitution products and identify the relationship between them.b)Identify which substitution product is expected to be favored, and explain why.c)Draw all elimination products, and identify which products are stereoisomers.d)For each pair of stereoisomericalkenes,identify which stereoisomer is expected to be favored.
Answers
a. Product 2 is formed when the ethyl group in Product 1 is replaced by a hydrogen atom.
b. The substitution product that is expected to be favored is Product 1, Ethylcyclohexane.
c. Product 3, Product 4, Product 5, Product 6, Product 7. Products 4 and 5, as well as Products 6 and 7, are stereoisomers of each other.
d. Product 7 is the only trans-1,3-diethylcyclohexene and is the only product of its kind, so it is favored by default.
The given tertiary alkyl halide was subjected to elimination reactions in ethanol, resulting in a mixture of five different elimination products and two substitution products. Let's take a closer look at each of the products.
a) The two substitution products can be drawn as follows:
- Product 1: Ethylcyclohexane
- Product 2: Cyclohexene
These two products are related by the fact that Product 2 is derived from the elimination of a hydrogen atom from one of the carbons in Product 1. In other words, Product 2 is formed when the ethyl group in Product 1 is replaced by a hydrogen atom.
b) This is because the elimination of a hydrogen atom from a tertiary carbon atom requires a strong base and high temperatures. In the given reaction conditions (ethanol, several days), elimination from a tertiary carbon is less favorable than substitution.
c) The five elimination products can be drawn as follows:
- Product 3: 1-Ethylcyclohexene
- Product 4: cis-1,2-Diethylcyclohexene
- Product 5: trans-1,2-Diethylcyclohexene
- Product 6: cis-1,3-Diethylcyclohexene
- Product 7: trans-1,3-Diethylcyclohexene
Products 4 and 5, as well as Products 6 and 7, are stereoisomers of each other.
d) In general, the favored stereoisomer in elimination reactions is the more substituted alkene. This is because elimination reactions follow Zaitsev's rule, which states that the major product is the more substituted alkene. Therefore, in this case:
- Products 3 and 5 are stereoisomers of each other, and the trans isomer (Product 5) is favored.
- Products 4 and 6 are stereoisomers of each other, and the cis isomer (Product 4) is favored.
- Product 7 is the only trans-1,3-diethylcyclohexene and is the only product of its kind, so it is favored by default.
To know more about alkyl halide visit:
brainly.com/question/31831733
#SPJ11
The atomic weight of hydrogen is 1.008 amu. What is the percent composition of hydrogen by isotope, assuming that hydrogen's only isotopes are 1H and 2D?
A. 92% H, 8% D
B. 99.2% H, 0.8% D
C. 99.92% H, 0.08% D
D. 99.992% H, 0.008% D
Answers
The percent composition of hydrogen by isotope, assuming that hydrogen's only isotopes are 1H and 2D, is 99.2% H and 0.8% D. (B)
1. The atomic weight of hydrogen is given as 1.008 amu.
2. The isotopes of hydrogen are 1H (with a mass of 1 amu) and 2D (with a mass of 2 amu).
3. To find the percent composition, we need to determine the relative abundance of each isotope.
4. Since the atomic weight is an average of the isotopic masses weighted by their abundance, we can set up an equation: (1 * x) + (2 * (1-x)) = 1.008, where x represents the relative abundance of 1H.
5. Solving for x, we get x = 0.992.
6. The relative abundance of 2D is 1-x = 0.008.
7. Convert these abundances to percentages: 1H is 99.2% and 2D is 0.8%.(B)
To know more about relative abundance click on below link:
https://brainly.com/question/1594226#
#SPJ11
what fraction of the 40k that was on earth when it formed 4.5 ✕ 109 years ago is left today? The half life of 40K is 1.25 × 109 years.
Answers
Approximately 6.25% of the original ⁴⁰K that was present on Earth when it formed 4.5 × 10⁹ years ago is left today.
The half-life of ⁴⁰K is 1.25 × 10⁹ years, which means that after 1.25 × 10⁹ years, half of the original amount of ⁴⁰K will decay. After another 1.25 × 10⁹ years, half of what remains will decay, and so on. Using this information, we can calculate the fraction of ⁴⁰K that is left today.
Let's define the original amount of ⁴⁰K as 1. Then after 1.25 × 10⁹ years, half of it will remain, which is 0.5. After another 1.25 × 10⁹ years, half of that will remain, which is 0.25. Continuing in this way, we can calculate the amount of ⁴⁰K that is left today as:
1 × (1/2)⁴ = 1/16
Therefore, the fraction of ⁴⁰K that is left today is 1/16 or approximately 6.25% of the original amount.
learn more about Half- life here:
https://brainly.com/question/24710827
#SPJ11
A solution that is 0.205 M in CH3NH2 and 0.100 M in CH3NH3Br. Solve an equilibrium problem ( using an ICE table) to calculate the pH of each solution
Answers
The pH of the solution which has 0.205 M CH₃NH₂ and 0.100 M in CH₃NH₃Br in is 11.59.
The reaction involved is
CH₃NH₂ + H₂O ⇌ CH₃NH₃+ + OH⁻
The equilibrium constant expression for this reaction is
Kb = ([CH₃NH₃⁺][OH⁻])/[CH₃NH₂]
The Kb for CH₃NH₂ is 4.4 × 10⁻⁴ at 25°C.
To solve the problem, we can set up an ICE table attached
Substituting the equilibrium concentrations into the Kb expression, we get
4.4 × 10⁻⁴ = (0.100 + x) × x / (0.205 - x)
Simplifying and solving for x, we get
x = 2.6 × 10⁻⁴ M
Therefore, [OH⁻] = [CH₃NH₃⁺] = 2.6 × 10⁻⁴ M
The pH of the solution can be calculated using the equation
pH = 14 - pOH
pH = 14 - (-log10[OH-])
pH = 11.59
To know more about pH here
https://brainly.com/question/2288405
#SPJ4
Convert 1. 709 x 10-5 cm3 to μm3 and express your answer with the correct number of significant figures
Answers
To convert 1.709 x 10^(-5) cm³ to μm³, we need to know the conversion factor between cm³ and μm³.
1 cm is equal to 10,000 μm since 1 cm = 10 mm and 1 mm = 1000 μm. Therefore, 1 cm³ is equal to (10,000 μm)³.
Calculating the conversion factor:
(10,000 μm)³ = 1,000,000,000,000 μm³
Now, we can convert the given value:
1.709 x 10^(-5) cm³ * 1,000,000,000,000 μm³ / 1 cm³ = 1.709 x 10^(-5) x 1,000,000,000,000 μm³ / 1 = 1.709 x 10^7 μm³
Since the given value has 4 significant figures (1.709), we need to express the final answer with the same number of significant figures. Therefore, the converted value of 1.709 x 10^(-5) cm³ to μm³, with the correct number of significant figures, is approximately 1.709 x 10^7 μm³.
Learn more about factor between cm³ and μm³ here
https://brainly.com/question/13980344
#SPJ11
1. record the temperature of the saturated borax solution.
Answers
To record the temperature of the saturated borax solution, you will need to use a thermometer to measure the temperature of the solution. Simply dip the thermometer into the solution and read the temperature. It is important to note that the temperature can affect the solubility of borax, so it is important to maintain a consistent temperature when working with this solution.
To record the temperature of the saturated borax solution, please follow these steps:
1. Prepare a saturated borax solution by dissolving borax in water until no more borax can dissolve, and the solution reaches a state of saturation.
2. Allow the solution to sit undisturbed for a few minutes to ensure even temperature distribution.
3. Using a clean and calibrated thermometer, insert the thermometer into the saturated borax solution, making sure it is fully submerged but not touching the bottom or sides of the container.
4. Wait for the temperature reading on the thermometer to stabilize, which typically takes about 30 seconds to 1 minute.
5. Once the temperature reading is stable, record the temperature of the saturated borax solution as indicated on the thermometer. Make sure to note the unit of measurement (e.g., Celsius or Fahrenheit).
Learn more about the temperature at https://brainly.com/question/14820864
#SPJ11
Calculate the pH of each solution.
A. [H3O+] = 7.7×10−8 M
B. [H3O+] = 4.0×10−7 M
C. [H3O+] = 3.2×10−6 M
D. [H3O+] = 4.4×10−4 M
Answers
The pH values for the solutions are: A: 7.11, B: 6.40, C: 5.49, D: 3.36
The pH scale is a measure of the acidity or basicity of a solution, with pH 7 being neutral, pH less than 7 being acidic, and pH greater than 7 being basic.
In the given formula, [H3O+] represents the concentration of hydronium ions in the solution, which is an indication of the acidity of the solution.
To calculate the pH of each solution, we take the negative logarithm (base 10) of the hydronium ion concentration. The lower the hydronium ion concentration, the higher the pH value, indicating a more basic solution.
Conversely, the higher the hydronium ion concentration, the lower the pH value, indicating a more acidic solution.
To calculate the pH of each solution, we will use the formula:
pH = -log10[H3O+]
A. pH = -log10(7.7×10−8 M) = 7.11
B. pH = -log10(4.0×10−7 M) = 6.40
C. pH = -log10(3.2×10−6 M) = 5.49
D. pH = -log10(4.4×10−4 M) = 3.36
So, the pH values for the solutions are:
A: 7.11
B: 6.40
C: 5.49
D: 3.36
To learn more about solution, refer below:
https://brainly.com/question/30665317
#SPJ11
What mass of ca(no 3 ) 2 is equal to 0.75 moles of this substance?
Answers
To calculate the mass of Ca(NO3)2 that is equal to 0.75 moles of this substance, we need to use the molar mass of Ca(NO3)2, which is 164.1 g/mol. We can use the following formula: mass = moles x molar mass
Plugging in the given values:
mass = 0.75 moles x 164.1 g/mol
mass = 123.075 g
Therefore, the mass of Ca(NO3)2 that is equal to 0.75 moles of this substance is 123.075 g.
To determine the mass of Ca(NO3)2 that is equal to 0.75 moles of this substance, follow these steps:
1. First, find the molar mass of Ca(NO3)2. To do this, add the molar masses of each element in the compound:
- Ca: 40.08 g/mol
- N: 14.01 g/mol (there are 2 nitrogen atoms, so multiply by 2)
- O: 16.00 g/mol (there are 6 oxygen atoms, so multiply by 6)
2. Calculate the molar mass of Ca(NO3)2:
- 40.08 + (2 x 14.01) + (6 x 16.00) = 40.08 + 28.02 + 96.00 = 164.10 g/mol
3. Now, multiply the given moles (0.75 moles) by the molar mass of Ca(NO3)2 to find the mass:
- Mass = 0.75 moles x 164.10 g/mol = 123.075 g
So, the mass of Ca(NO3)2 that is equal to 0.75 moles of this substance is 123.075 grams.
To know more about Mass visit:
https://brainly.com/question/19694949
#SPJ11
The mass of 0.75 moles of the given compound ca(NO₃)₂ is determined as 123 g.
What mass of ca(NO₃)₂ is equal to 0.75 moles of this substance?The molar mass of ca(NO₃)₂ is calculated as follows;
molar mass = 40 + (2 x 14 ) + (16 x 3 x 2) = 164 g/mol
The mass of 0.75 moles of the given compound ca(NO₃)₂ is calculated by applying the following formula;
1 mole of the substance = 164 g
0.75 moles of the substance = ?
= ( 0 . 75 x 164 ) / 1
= 123 g
Thus, the mass of 0.75 moles of the given compound ca(NO₃)₂ is determined as 123 g.
Learn more about reactant mass here: https://brainly.com/question/26682140
#SPJ4
what is the iupac name for the following compound? group of answer choices 2-methylhexanoic acid none of these 3-methylhexanoic acid 2−methylpentanoic acid 3-methylpentanoic acid
Answers
The IUPAC name for the given compound is 3-methylhexanoic acid. To arrive at this name, we need to follow a few rules laid down by the IUPAC. Firstly, we need to identify the longest carbon chain in the compound, which contains the functional group (-COOH) and number the carbons in the chain accordingly. Here, we can see that the longest chain has six carbons, so it is a hexanoic acid. Next, we need to identify and name any substituents attached to the main chain. In this compound, we have a methyl group attached to the third carbon, so it becomes 3-methylhexanoic acid. Therefore, the correct IUPAC name for the given compound is 3-methylhexanoic acid. It is important to use correct IUPAC names for compounds to avoid confusion and ensure that everyone is referring to the same molecule.
The IUPAC name for the given compound is 3-methylhexanoic acid. In this compound, the methyl group is attached to the third carbon in the hexanoic acid chain, which consists of six carbon atoms. When numbering the carbon atoms, start from the carboxyl group (COOH) as carbon 1, and count along the chain. The methyl group is attached to the third carbon, resulting in the name 3-methylhexanoic acid.
The negative muon has a charge equal to that of an electron but a mass that is 207 times as great. Consider a hydrogenlike atom consisting of a proton and a muon. (a) What is the reduced mass of the atom? (b) What is the ground-level energy (in electron volts)? (c) What is the wavelength of the radiation emitted in the transition from the n = 2 level to the n = 1 level?
Answers
If we consider a hydrogenlike atom consisting of a proton and a muon, (a) The reduced mass is 206.93 times the mass of an electron. (b) The ground-level energy is 13.6 eV) (c) The wavelength is approximately 1.22 nanometers (nm).
(a) The reduced mass (μ) of a hydrogenlike atom is calculated using the formula:
μ = (m₁ * m₂) / (m₁ + m₂)
where m₁ and m₂ are the masses of the two particles. Given that the mass of the muon is 207 times that of an electron, the reduced mass is approximately 206.93 times the electron mass.
(b) The ground-level energy of a hydrogenlike atom can be determined using the Rydberg formula:
E = -13.6 eV / n²
where n is the principal quantum number. For the ground state, n = 1, so the ground-level energy is -13.6 eV.
(c) The wavelength (λ) of the radiation emitted in a transition between energy levels is given by the Rydberg formula:
1/λ = [tex]R_H[/tex] * (1/n₁² - 1/n₂²)
where [tex]R_H[/tex] is the Rydberg constant for hydrogen and n₁ and n₂ are the principal quantum numbers of the initial and final levels, respectively. For the transition from n = 2 to n = 1, plugging the values into the formula gives a wavelength of approximately 1.22 nm.
To know more about Rydberg formula, refer here:
https://brainly.com/question/13185515#
#SPJ11
Determine whether the following compounds are organometallic. Explain your answer. (i) Cacz (ii) CH3COONa (iii) Cr(CO) (iv) B(C2H5)3
Answers
Cacz includes a carbon-metal link, making it an organometallic compound (i). It is an organometallic complex since the element Ca is a metal and is covalently joined to the carbon atom.
(ii) Since CH3COONa lacks a direct carbon-metal connection, it is not an organometallic compound. Na is a metal, but the carbon atoms in the acetate ion are not chemically bound to it.
Cr(CO), which has a carbon-metal link, is an organometallic compound (iii). It is an organometallic molecule because the metal Cr is covalently joined to the carbon monoxide (CO) ligands.
B(C2H5)3 is an organometallic compound since it has a carbon-metal bond. It is an organometallic compound because the metalloid element B is covalently linked to the carbon atoms in the ethyl groups.
For more such question on organometallic
https://brainly.com/question/13428382
#SPJ11
Out of the four given compounds, only B(C_{2}H_{5})_{3} is organometallic. Organometallic compounds are compounds that contain a covalent bond between a carbon atom and a metal atom. In the case of B(C_[2}H_{5})_{3}, there is a covalent bond between a boron atom and three ethyl (C_{2}H_{5}) groups. This makes it an organometallic compound.
Cacz, CH_{3}COONa, and Cr(CO) are not organometallic compounds. Cacz is calcium carbide, which is a simple ionic compound and does not contain any covalent bonds between carbon and metal atoms. CH_{3}COONa is sodium acetate, which is a salt that does not contain any metal atoms. Cr(CO) is a metal carbonyl complex, but it does not have a direct covalent bond between carbon and chromium atoms.In summary, only B(C_{2}H_{5})_{3} is an organometallic compound as it contains a covalent bond between a carbon atom and a boron atom, while the other compounds do not have this feature.
learn more about organometallic refer: https://brainly.com/question/30655091
#SPJ11
Calculate the value of AGº (in kJ) for the following reaction3 NO(g) -> N2O(g) + NO2(g), using the values of ΔGfº (in kJ/mol) given below.• ΔGfº (NO) = 84 • ΔGfº (NO2) = 48 • ΔGfº (N20) = 107 Enter value as an integer (value + 2)
Answers
The value of AGº for the reaction 3 NO(g) -> N2O(g) + NO2(g) is -50 kJ (84 + 48 - 3*107 = -50). To calculate the standard free energy change (ΔGº) for a reaction, we use the formula:
ΔGº = ΣnΔGfº(products) - ΣmΔGfº(reactants)
Where n and m are the stoichiometric coefficients of the products and reactants, respectively. ΔGfº is the standard free energy of formation, which is the free energy change when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm pressure).
Using the given values of ΔGfº for NO, NO2, and N2O, we can substitute them in the above formula to get the value of ΔGº for the reaction.
ΔGº = [1ΔGfº(N2O) + 1ΔGfº(NO2)] - [3*ΔGfº(NO)]
Substituting the values, we get:
ΔGº = [1*(107) + 1*(48)] - [3*(84)]
ΔGº = -50 kJ
A negative value for ΔGº indicates that the reaction is thermodynamically favorable, meaning that it can occur spontaneously.
learn more about reaction here:
https://brainly.com/question/28984750
#SPJ11
How does the volume of 1 mol of an ideal gas change if the temperature and the pressure are both decreased by a factor of four?a) decreases by four times.b) decreases by sixteen times.c) increases by four times.d) increases by sixteen times.e) remains unchanged.
Answers
To determine how the volume of 1 mol of an ideal gas changes when both the temperature and pressure are decreased by a factor of four, we will use the Ideal Gas Law equation:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature.
Initially, let the volume be V1, the pressure be P1, and the temperature be T1. After decreasing the temperature and pressure by a factor of four, let the new volume be V2,
the new pressure be P2 (P1/4), and the new temperature be T2 (T1/4).
Using the Ideal Gas Law for both initial and final conditions:
P1 * V1 = nRT1
(P1/4) * V2 = nR(T1/4)
Now, divide the second equation by the first equation:
(V2 / V1) = (P1 / (P1/4)) * (T1/4 / T1)
Simplifying the equation, we get:
(V2 / V1) = (4) * (1/4)
(V2 / V1) = 1
Therefore, the volume remains unchanged. So, the answer is (e) remains unchanged.
To know more about ideal gas refer here
https://brainly.com/question/31463642#
#SPJ11
Oil is sometimes found trapped beneath a ‘cap’. Shale is good at reflecting sound waves underground. Why does this mean that geophysicists must scan the rocks with sound waves from different points?
Answers
Geophysicists use sound waves to scan rocks from different points because shale, which is good at reflecting sound waves underground, can create a barrier or "cap" that traps oil beneath it. By scanning the rocks from different angles and points, geophysicists can gather more comprehensive data and identify the location and extent of the trapped oil.
Shale is a type of sedimentary rock that has a high capacity for reflecting sound waves. When oil is present beneath the shale, it acts as a barrier or cap that prevents the oil from migrating further. To locate and assess the potential oil reservoir, geophysicists use a technique called seismic reflection, which involves sending sound waves into the ground and analyzing the reflected waves.
By scanning the rocks from different points or angles, geophysicists can obtain multiple sets of seismic data that provide a more complete picture of the subsurface structure. This allows them to analyze the reflections and variations in the sound waves, which can indicate the presence of oil traps or reservoirs. By combining the data from different points, geophysicists can create a three-dimensional model of the subsurface and make more accurate predictions about the location and extent of the oil reservoirs.
Learn more about Geophysicists here:
https://brainly.com/question/32469429
#SPJ11
2hbr(g)h2(g) br2(l) using standard absolute entropies at 298k, calculate the entropy change for the system when 1.83 moles of hbr(g) react at standard conditions. s°system = j/k
Answers
The entropy change for system when 1.83 moles of HBr reacts at standard condition = -- 104.76 k/j .
Evaluating entropy change :ΔS°r×n = ΔS°product - ΔS°reactant
= 130 .7 + 152.2 - 2 ×[198.7]
= - 114.5 J / K
2 mol of HBr ⇒ - 114.5 j/k
1. 83 mol of HBr ⇒ -114.5 × 1.83 /2
ΔS°system = -- 104.76 j/k
Entropy Change :It is the peculiarity which is the proportion of progress of turmoil or irregularity in a thermodynamic framework. It is connected with the transformation of intensity or enthalpy accomplished in work. Entropy is high in a thermodynamic system with more randomness.
What is unit of enthalpy?Enthalpy is a state function or property that has the dimensions of energy and is therefore measured in joules or ergs. Its value is entirely determined by the system's temperature, pressure, and composition, not by the system's history.
Learn more about entropy change :
brainly.com/question/27549115
#SPJ4
he vapor pressure of water at 80°c is 355.torr. calculate the vapor pressure in mmhg and atm. round each of your answers to 3 significant digits.
Answers
The vapor pressure of water at 80°C is 355 torr. We need to calculate the vapor pressure in mmHg and atm.
To convert torr to mmHg, we simply need to multiply the value in torr by 1 mmHg/1 torr.
So, the vapor pressure in mmHg can be calculated as:
355 torr x (1 mmHg/1 torr) = 355 mmHg
To convert torr to atm, we need to divide the value in torr by 760 torr/atm. So, the vapor pressure in atm can be calculated as:
355 torr ÷ 760 torr/atm = 0.467 atm
We need to round each answer to 3 significant digits, so the vapor pressure in mmHg is 355 mmHg and the vapor pressure in atm is 0.467 atm.
The vapor pressure of water at 80°C is 355 torr, which is equivalent to 355 mmHg and 0.467 atm.
To know more about vapor pressure, visit:
https://brainly.com/question/11864750
#SPJ11
Consider the reaction represented by the following chemical equation: A(g) = 2B (g) K = 10.0 at 300K If a flask is filled with 0.200 atm of A (g) and 0.100 atm of B(8) at 300K, what would the partial pressure (in atm) of B (g) be when the reaction mixture reaches equilibrium? Assume that both the volume and temperature of the flask remain constant. Report your answer to at least three significant figures
Answers
The equilibrium constant expression for the reaction is K = [B]^2 / [A] he partial pressure of B at equilibrium is 0.2344 atm.
In chemistry, equilibrium refers to a state of balance in which the forward and reverse reactions of a chemical reaction occur at the same rate. At equilibrium, the concentrations of reactants and products remain constant over time, although the individual molecules are constantly undergoing reactions.Equilibrium is governed by the equilibrium constant, K, which is defined as the ratio of the concentration of products to the concentration of reactants, with each concentration raised to a power equal to the stoichiometric coefficient of the species in the balanced chemical equation. The value of K depends only on the temperature of the system, and is a measure of the position of the equilibrium.
To know more about equilibrium visit :
https://brainly.com/question/30807709
#SPJ11
Which option does NOT demonstrate a
property of heat?
A. A physical substance.
B. The KE of molecules.
C. A form of energy transfer.
D. It is a form of energy. helllllllllppppppp
Answers
The option that does not demonstrate a property of heat is that it is a physical substance (option A).
What is heat?Heat is the transfer of kinetic energy from one medium or object to another, or from an energy source to a medium or object.
Heat can also refer to the thermal energy transferred between two systems at different temperatures that come in contact.
Heat is a form of energy and not a physical substance. Therefore, the first option is the correct answer.
Learn more about heat at: https://brainly.com/question/25384702
#SPJ1
Red phosphorus reacts with liquid bromine in an exothermic reaction, 2P(s)+3Br 2
(l)→2PBr 3
(g):Δ r
H o
=−243 kJ. Calculate the enthalpy change when 2.63 g of phosphorus reacts with an excess of bromine in this way.
Answers
The enthalpy change when 2.63 g of phosphorus reacts with an excess of bromine is -20.6 kJ, indicating an exothermic reaction where heat is released.
To calculate the enthalpy change when 2.63 g of phosphorus reacts with an excess of bromine, we need to use stoichiometry and the given enthalpy change of the reaction.
First, we need to convert the mass of phosphorus to moles:
moles of P = mass of P / molar mass of P
moles of P = 2.63 g / 30.97 g/mol
moles of P = 0.0849 mol
Next, we can use the balanced chemical equation to determine the moles of bromine consumed in the reaction. According to the equation, 2 moles of P react with 3 moles of Br2, so:
moles of Br2 = (3/2) x moles of P
moles of Br2 = (3/2) x 0.0849 mol
moles of Br2 = 0.1273 mol
Finally, we can use the enthalpy change of the reaction to calculate the total heat released in the reaction:
ΔH = moles of PBr3 x ΔH of the reaction
ΔH = (0.0849 mol PBr3) x (-243 kJ/mol)
ΔH = -20.6 kJ
To know more about exothermic reaction refer here :-
https://brainly.com/question/28546817#
#SPJ11
report form: mixed aldol conednsations of benzaldehyde and acetone Part A Balanced Equation(s) for Main Reaction(s): mmol compound benzaldehyde MW 106.12 58.08 mg or ml 1.00ml 0.36m1 9.84 4.9 *acetone 40.00 sodium hydroxide 0.025 1000mg 43mg product A Indicate the limiting reagent with an asterisk (*). Product 110oc Observed melting point range: Literature melting point range:- °C Molecular weight of product: Theoretical yield: Grams obtained: % Experimental yield: 8 126 Name: REPORT FORM: MIXED ALDOL CONDENSATIONS OF BENZALDEHYDE AND ACETONE Part B Balanced Equation(s) for Main Reaction(s): mmol compound mg or ml benzaldehyde MW 106.12 58.08 140.00 0.5ml 3.00ml acetone sodium hydroxide 230my 773mg product A Indicate the limiting reagent with an asterisk (*). Product Observed melting-point range: LOC Literature melting-point range: °C Molecular weight of product: Theoretical yield: 8 Grams obtained: Experimental yield: %
Answers
The limiting reagent is acetone, as it is present in the smallest quantity (230 mg). The observed melting-point range of the product is not given, but the literature melting-point range is provided.
The balanced equation for the main reaction in Part A of the mixed aldol condensation of benzaldehyde and acetone is:
2 benzaldehyde + acetone + NaOH → product A
The limiting reagent is benzaldehyde, as it is the one present in the smallest quantity (0.36 mmol). The observed melting point range of the product is 110°C, while the literature melting point range is not provided. The molecular weight of the product is not given either, but the theoretical yield can be calculated by using the limiting reagent (benzaldehyde) and assuming a 100% yield. The theoretical yield is 9.84 mg, but the actual grams obtained and experimental yield are not provided.
In Part B, the balanced equation for the main reaction is:
3 benzaldehyde + 2 acetone + 2 NaOH → product A
The limiting reagent is acetone, as it is present in the smallest quantity (230 mg). The observed melting-point range of the product is not given, but the literature melting-point range is provided. The molecular weight of the product is not provided either, but the theoretical yield can be calculated using the limiting reagent (acetone) and assuming a 100% yield. The theoretical yield is 8 grams, but the actual grams obtained and experimental yield are not provided.
To know more about benzaldehyde visit:
https://brainly.com/question/31684857
#SPJ11
when we titrate oxalate ions with permanganate ions, why is the iron(iii) ion of our complex not also oxidized?
Answers
When we titrate oxalate ions with permanganate ions, the iron (III) ion of our complex is not oxidized because it is not susceptible to oxidation by permanganate ions. This is because the iron (III) ion is already in its highest oxidation state and is relatively stable in that state. The oxidation state of the iron ion in the complex is +3, which means that it has already lost three electrons and is highly oxidized.
Permanganate ions are powerful oxidizing agents, and they have a high tendency to oxidize other substances that are susceptible to oxidation. In the case of oxalate ions, they have a relatively low oxidation state, and they are susceptible to oxidation by permanganate ions. Therefore, the permanganate ions oxidize the oxalate ions, causing a color change in the solution from pink to colorless.
In conclusion, the iron (III) ion of our complex is not oxidized during the titration of oxalate ions with permanganate ions because it is already in its highest oxidation state, and it is relatively stable in that state. The oxidation of oxalate ions occurs due to their low oxidation state, which makes them susceptible to oxidation by permanganate ions.
To know more about permanganate ions visit:
https://brainly.com/question/30572418
#SPJ11
In the reaction 2Cr+3Ni 2+
→3Ni+2Cr 3+
, the species oxidized is:
Answers
The species that has been oxidized in this reaction is chromium (Cr).
In the given reaction, the oxidation state of chromium changes from +2 to +3, while the oxidation state of nickel changes from +3 to +2. This indicates that chromium has lost electrons and nickel has gained electrons. Therefore, chromium is the species that has been oxidized in this reaction.
To further explain, oxidation is defined as the loss of electrons or an increase in oxidation state, while reduction is defined as the gain of electrons or a decrease in oxidation state. In this reaction, the oxidation state of chromium has increased from +2 to +3, indicating that it has lost electrons and has been oxidized. On the other hand, the oxidation state of nickel has decreased from +3 to +2, indicating that it has gained electrons and has been reduced.
Therefore, the species that has been oxidized in this reaction is chromium (Cr).
To know more about chromium click here:
https://brainly.com/question/681602
#SPJ11
A solid with a mass of 200g at its melting point temperature in a coffee cup calorimeter. While the substance changes from a solid to a liquid at the asme temperature of 30 degrees C.
a) How much heat did the water lose while the substance melted?
b) What is the heat of the fusion of the substance that melted?
c) If the substance has a molar mass of 16.35 g/mol, calculate the kilojuoles required to melt 3.28 mol of the substance
Answers
a) The water lost 6,600 J of heat while the substance melted.
b) The heat of fusion of the substance is 33 J/g.
a) To calculate how much heat the water lost while the substance melted, we need to use the formula Q = m * ΔH, where Q is the heat lost, m is the mass of water, and ΔH is the heat of fusion of the substance. Since the substance melted at 30 degrees C, we assume that the water also lost heat to cool down to that temperature. Assuming the specific heat capacity of water is 4.184 J/g·°C, we can calculate that the water lost 1,580 J to cool down to 30 degrees C. Therefore, the water lost 6,600 J of heat while the substance melted.
b) The heat of fusion of the substance can be calculated by using the formula Q = m * ΔH, where Q is the heat lost, m is the mass of the substance, and ΔH is the heat of fusion. Substituting the given values, we get ΔH = Q / m = 6,600 J / 200 g = 33 J/g.
c) To calculate the kilojoules required to melt 3.28 mol of the substance, we first need to calculate the mass of the substance. Using the molar mass given (16.35 g/mol), we get 3.28 mol * 16.35 g/mol = 53.718 g. Then, we can use the formula Q = m * ΔH, where ΔH is the heat of fusion calculated in part b. Substituting the values, we get Q = 53.718 g * 33 J/g = 1,773.294 J. Converting this to kilojoules, we get 1.773 kJ.
To know more about the heat visit:
https://brainly.com/question/10763306
#SPJ11
in preparing a series of standards for calibration of colorimeter a stock solution of 0.100 m niso4 (molar mass =154.76g/mol) solution was required . to prepare this stock solution7.74 g of NiSO4 should be added to a 500.0mL volumetric flask and the volume made up to the calibration mark with deionized water. 07.74g of NiSO4 should be added to 500.0 mL of deionized water in a volumetric flask. 15.5 g of NISO4 should be added to 500.0 mL of deionized water in a volumetric flask. 15.5 g of NiSO4 should be added to a 500.0 mL volumetric flask and the volume made up to the calibration mark with deionized water. 15.5g of NiSO4 should be added to 500.0 mL of deionized water in a beaker.
Answers
To prepare a 0.100 M NiSO4 stock solution for the calibration of a colorimeter,7.74g of NiSO₄ should be added to a 500.0 mL volumetric flask and make up the volume to the calibration mark with deionized water.
The step-by-step explanation:
1. Calculate the mass of NiSO₄ needed for a 0.100 M solution in 500.0 mL:
= (0.100 mol/L) x (154.76 g/mol) x (0.500 L) = 7.74 g
2. Weigh out 7.74 g of NiSO₄ using a balance.
3. Add the 7.74 g of NiSO₄ to a clean 500.0 mL volumetric flask.
4. Add deionized water to the volumetric flask, filling it up to the calibration mark. This ensures you have exactly 500.0 mL of solution.
5. Mix the solution thoroughly to ensure the NiSO₄ is completely dissolved in the deionized water.
Thus, 0.100 M NiSO₄ stock solution is prepared that can be used for the calibration of your colorimeter.
To learn more about calibration visit:
https://brainly.com/question/525672
#SPJ11
3. What is the molar mass of baking soda? Show your work.
4. How many moles of baking soda does the recipe call for? Show your work.
5. What’s the difference between the mass of baking soda and the moles of baking soda? Explain
Answers
The molar mass of baking soda (sodium bicarbonate) is approximately 84.01 g/mol. The recipe calls for a certain number of moles of baking soda, which can be calculated using the molar mass and the given mass of baking soda.
To determine the molar mass of baking soda ([tex]NaHCO_{3}[/tex]), we add up the atomic masses of its constituent elements. The atomic mass of sodium (Na) is approximately 22.99 g/mol, hydrogen (H) is 1.01 g/mol, carbon (C) is 12.01 g/mol, and oxygen (O) is 16.00 g/mol. Adding these masses together:
Molar mass of NaHCO_{3} = (22.99 g/mol) + (1.01 g/mol) + (12.01 g/mol) + (3 * 16.00 g/mol) ≈ 84.01 g/mol
To calculate the number of moles of baking soda required by the recipe, we divide the given mass of baking soda by its molar mass. The mass is not provided in the question, so the calculation cannot be performed without additional information.
The difference between the mass of baking soda and the moles of baking soda lies in their units. Mass is measured in grams (g), while moles represent a quantity of particles. The number of moles is obtained by dividing the mass of the substance by its molar mass. Essentially, moles provide a way to count the number of entities (atoms, molecules) in a given sample, whereas mass represents the total amount of matter present.
Learn more about moles here: https://brainly.com/question/29367909
#SPJ11