Answers
Answer:
Explanation:
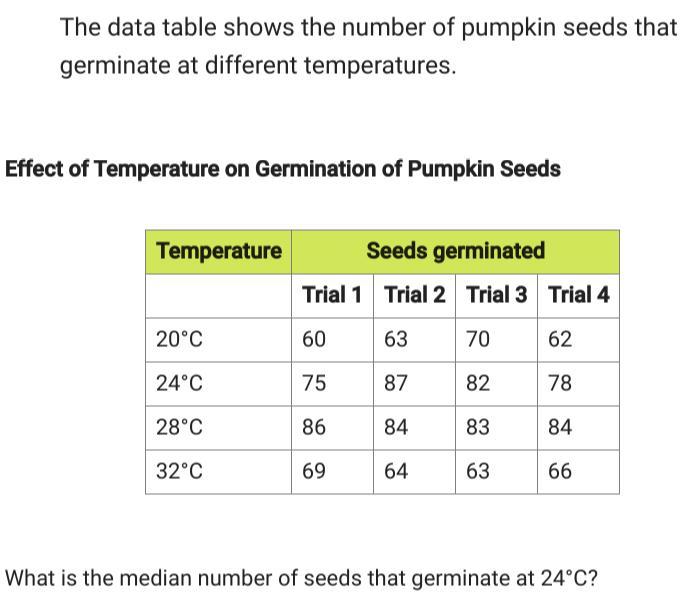
80
Related Questions
When drawing the Lewis structure of a molecule, start by determining the total number of available valence based on each element's ___________ group number. Then, use the total number of electrons needed for each element to be stable, generally based on________ its charge, to determine the____________ ionic charge by finding the difference between the number of needed and available electrons divided by two.
Next, identify the central atom, which is the element with the __________ fewest valence electrons other than hydrogen. Finally, arrange the number of bonds around the central atom to fulfill the stable number of electrons for each element.
Answers
Answer:
Group number, octet's rule, total number of bonds and fewest valence electrons.
Explanation:
Hello,
For the given statement is answer is bolded:
"When drawing the Lewis structure of a molecule, start by determining the total number of available valence based on each element's group number. Then, use the total number of electrons needed for each element to be stable, generally based on the octet's rule its charge, to determine the total number of bonds by finding the difference between the number of needed and available electrons divided by two.
Next, identify the central atom, which is the element with the fewest valence electrons other than hydrogen. Finally, arrange the number of bonds around the central atom to fulfill the stable number of electrons for each element".
For the first one, it is widely known that the group number provides the number of valence electrons as nitrogen is in group VA and it has five valence electrons, chlorine is in grou´p VIIA and it has seven valence electrons and so on.
For the second one, it is also known that the octet's rule limit the amount of bonds as it has been demonstrated that each compound can hold up to 8 electrons overall excluding some exceptions.
For the third one, based on the octet's rule, an element must have as much bonds as missing electrons to complete eight, for instance, carbon has four valence electrons, so it need four bonds (each one providing one valence electron) in order to attain the octet.
Finally, the central atom must have the fewest number of valence electrons as it shows the other bonds and elements attaining the octet. Usually, the central atom is not demanded to get 8 electrons, for instance in AlCl₃, which is:
[tex]\ \ \ \ \ Cl-Al-Cl\\.\ \ \ \ \ \ \ \ \ \ \ \ \ |\\.\ \ \ \ \ \ \ \ \ \ \ \ Cl[/tex]
Has aluminum as the central one due to the fact that it has three valence electrons whereas chlorine seven and it does not attain the octet.
Best regards.
what is so unique about water that hydrogen bonding becomes possible
Answers
Answer:
Water molecules are polar, so they form hydrogen bonds. This gives water unique properties, such as a relatively high boiling point, high specific heat, cohesion, adhesion and density.
EXPLANATION :
So it is unusual for water to be a liquid at room temperature! Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds resulting in the molecules being packed more closely together.
One way to represent a substance is with a chemical formula. In the formula CO2, what do the symbols Cand o refer to?
Answers
Answer:
C is for carbon and O is for oxygen
Has 121 nuetrons and 80 electrons plz help i will pick you as brainliest
Answers
Answer:
Mercury Atom
Explanation:
If you are talking about what has 121 neutrons and 80 electrons, then it would be a Mercury Atom. Hope this helps!
A sample of an ideal gas has a volume of 2.23 L at 289 K and 1.05 atm. Calculate the pressure when the volume is 1.08 L and the temperature is 304 K. P= atm
Answers
Answer:
2.28 atm
Explanation:
V₁ = 2.33L, V₂ = 1.08L
T₁ = 289K, T₂ = 304K
P₁ = 1.05 atm, P₂ = ?
Where V₁ and V₂ are initial and final volume respectively
T₁ and T₂ are initial and final temperature respectively
P₁ and P₂ are initial and final pressure respectively
The formula to be used here is the general gas equation:
P₁V₁/T₁=P₂V₂/T₂
1.05 × 2.23/289 = P₂ × 1.08/304
P₂ × 1.08 × 289 = 1.05 × 2.23 × 304
P₂ = (1.05 × 2.23 ×304) ÷ (1.08 × 289)
P₂ = 711.82 ÷ 312.12
P₂ = 2.28 atm
Use the data in the Successive Ionization Energies and Electron Affinity tables to determine the following. (Assume the values in the Successive Ionization Energies table are given to the ones place.) (a) the electron affinity of Ar2+ kJ/mol (b) the electron affinity of Si + kJ/mol (c) the ionization energy of Cl − kJ/mol (d) the ionization energy of Cl kJ/mol (e) the electron affinity of Cl + kJ/mol
Answers
Answer:
Use the data in the Successive Ionization Energies and Electron Affinity tables to determine the following. (Assume the values in the Successive Ionization Energies table are given to the ones place.)
(a)
the electron affinity of Ar2+
kJ/mol
(b)
the electron affinity of S+
kJ/mol
(c)
the ionization energy of Cl−
kJ/mol
(d)
the ionization energy of Cl
kJ/mol
(e)
the electron affinity of Cl+
kJ/mol
Successive Ionization Energies in Kilojoules per Mole for the Elements in Period 3 General increase 13 14 Element 11 12 I. 16
Explanation:
An ideal gaseous reaction (which is a hypothetical gaseous reaction that conforms to the laws governing gas behavior) occurs at a constant pressure of 35.0 atm and releases 74.6 kJ of heat. Before the reaction, the volume of the system was 8.20 L . After the reaction, the volume of the system was 2.80 L . Calculate the total internal energy change, ΔE, in kilojoules.
Answers
Answer:
ΔU = −55.45 kJ
Explanation:
From first law of thermodynamics in chemistry, we have;
ΔU = Q + W
where;
ΔU is change in internal energy
Q is the net heat transfer
W is the net work done
We are given;
Q = 74.6 kJ
But Q will be negative since heat is released
Thus;
ΔU = -74.6 kJ + W
We are given;
Constant pressure; P = 35 atm = 35 × 101325 = 3546375 N/m²
Volume before reaction; Vi = 8.2 L = 0.0082 m³
Volume after reaction; V_f = 2.8 L = 0.0028 m³
Now,
W = -P(V_f - V_i)
W = - 3546375(0.0028 - 0.0082)
W = 19.15 KJ
Thus;
ΔU = Q + W
ΔU = -74.6 kJ + 19.15 KJ =
ΔU = −55.45 kJ
The atoms of which element will gain electrons to form an ion?
oxygen
calcium
lithium
mercury
Answers
Explanation:
Oxygen will gain electrons to form ion (O²⁻).
Calcium loses electron to form ion (Ca²⁺)
Lithium loses electron to form ion (Li⁺)
Mercury loses electron to form ion (Hg²⁺)
Here it is stated in question that the element will gain electron it means electron bears negative charge.
Therefore,
Option A is correct ✔.
I need help on this. It’s kinda confusing...
Answers
Answer: here u go
Explanation:
A. 3.7 x 10^4
B. 4.56 × 10^-8
C. 8.01 × 10^6
Work for A:
Step 1
To find a, take the number and move a decimal place to the right one position.
Original Number: 37,000
New Number: 3.7000
Step 2
Now, to find b, count how many places to the right of the decimal.
New Number: 3 . 7 0 0 0
Decimal Count: 1 2 3 4
There are 4 places to the right of the decimal place.
Step 3
Building upon what we know above, we can now reconstruct the number into scientific notation.
Remember, the notation is: a x 10b
a = 3.7 (Please notice any zeroes on the end have been removed)
b = 4
Now the whole thing:
3.7 x 104
Step 4
Check your work:
104 = 10,000 x 3.7 = 37,000
Work for B:
Step 1
To find a, take the number and move a decimal place to the right one position.
Original Number: 456
New Number: 0.0000000456
Step 2
Now, to find b, count how many places to the right of the decimal.
New Number: 0 . 0 0 0 0 0 0 0 4 5 6
Decimal Count: 1 2 3 4 5 6 7 8 9 10
There are 2 places to the right of the decimal place.
Step 3
Building upon what we know above, we can now reconstruct the number into scientific notation.
Remember, the notation is: a x 10b
a = 4.56
b = 2
Now the whole thing:
4.56 x 102
Step 4
Check your work:
102 = 100 x 4.56 = 456
Work for C:
Step 1
To find a, take the number and move a decimal place to the right one position.
Original Number: 8,010,000
New Number: 8.010000
Step 2
Now, to find b, count how many places to the right of the decimal.
New Number: 8 . 0 1 0 0 0 0
Decimal Count: 1 2 3 4 5 6
There are 6 places to the right of the decimal place.
Step 3
Building upon what we know above, we can now reconstruct the number into scientific notation.
Remember, the notation is: a x 10b
a = 8.01 (Please notice any zeroes on the end have been removed)
b = 6
Now the whole thing:
8.01 x 106
Step 4
Check your work:
106 = 1,000,000 x 8.01 = 8,010,000
Hope this helps!
Write the half-reaction for ribose conversion to CO2. Is it an oxidation- or reduction- half reaction
Answers
Answer:
[tex]5H_2O+C_5H_{10}O_5\rightarrow 5CO_2+20H^++20e^-[/tex]
Explanation:
Hello.
In this case, when ribose (C₅H₁₀O₅) yields carbon dioxide (CO₂) we write:
[tex]C_5H_{10}O_5\rightarrow CO_2[/tex]
Which needs to be balanced by adding water and hydrogen ions:
[tex]5H_2O+C_5H_{10}O_5\rightarrow 5CO_2+20H^++20e^-[/tex]
You can also see that there are 20 transferred electrons, since the carbon atoms in the ribose have 0 as their oxidation state and the carbon atoms in the carbon dioxide have +4 as the oxidation state, thus, each carbon transfers 4 electrons, a five carbon atoms transfer 20 electrons overall.
In such a way, since the carbon is increasing its oxidation state, such half reaction is an oxidation half reaction.
Best regards.
A gas mixture contains HBr, NO2, and C2H6 at STP. If a tiny hole is made in the container, which gas will effuse fastest? NO2 C2H6 HBr They all effuse at the same rate. Which gas molecules have the highest average kinetic energy at this temperature? HBr NO2 C2H6 They all have the same average kinetic energy.
Answers
Answer:
C2H6
Explanation:
Let us first consider the molar Masses of each gas
HBr - 80.91 g/mol
NO2 - 46.0055 g/mol
C2H6 - 30.07 g/mol
We must remember that the greater the molar mass of a gas the lesser its velocity and average kinetic energy.
Looking at the gases listed, C2H6 have the highest average kinetic energy at this temperature since it has the lowest molecular mass. This reasoning is directly derived from Graham's law of diffusion in gases.
Hence C2H6 will effuse fastest when a hole is made in the container. It also possess the greatest average kinetic energy because it has the lowest molecular mass.
A student determines that the mass of a sample of aluminum is 8.3 g. How many moles are in the sample?
Answers
Answer:
0.307
Explanation:
use the formula of n=mass /molar mass
is used to locate and track severe storm
Answers
Answer:
Weather radar is used to locate and track severe storm.
H₂C=CH-CH₂-CH=CH₂
how many single and double bonds
Answers
Answer:
2 double bonds 2 single bonds
But the hydrogen atoms in this molecule are also covalently bonded to the carbon atoms. All the carbon–hydrogen bonds here are single bonds, and there are eight such bonds.
So, in sum, this molecule (1,4-pentadiene) has ten single bonds (two C–C single bonds and eight C–H single bonds) and two double bonds (both C=C).
What are the two ways
that heat is measured?
Answers
Answer:
heat is mesured in calories and also joules
Explanation:
Condensation occurs with the removal of thermal energy.
True or False? If false, rewrite to make the question true.
PLZZZ HELP
Answers
Answer:
true
Explanation:
Condensation is the opposite of vaporization. True. When does condensation occurs, does a gas lose or gain thermal energy?
Answer:
True
Explanation:
Condensation is formed with moisture and cold air, so the removal of thermal energy, or heat, would create the perfect conditions :)
Soil is an example of a:
a. solution
b. heterogeneous mixture
c. solid solution
Answers
Answer:
Heterogeneous mixture
Explanation:
Soil is composed of small pieces of a variety of materials, so it is a heterogeneous mixture.
Which accurately represents these building blocks of matter from the smallest to the largest?
atom -- molecule or compound
O molecule -- atom - element
compound - molecule -- element
molecule atom or element
Answers
Answer:
A - Atom ---> molecule or compound.
write the equation for the beta decay of 53/26 FE
Answers
Answer:
53/26Fe = 0/-1,B + 53/27 Co
Which element contains four electrons in its third and outer main energy level?
Answers
Answer:
valence electrons
Explanation:
The valence electrons are the outer most electrons and the principal energy level in which they belong will vary for .
The chemical element which contains four (4) electrons in its third and outer main energy level is: Silicon (Si).
An electron shell can be defined as the outermost shell of an atom of a chemical element around the atomic nucleus.
Hence, an electron shell is an orbital that is typically accompanied by electrons around the nucleus of an atom.
A sublevel is also referred to as an orbital and it can be defined as an energy level that is associated with the electrons found outside the atomic nucleus.
In Chemistry, there are four main (4) types of sublevel and these are:
s orbital (sublevel): it has one (1) orbital i.e 1s.p orbital (sublevel): it has three (3) orbitals.d orbital (sublevel): it has five (5) orbitals.f orbital (sublevel): it has seven (7) orbitals.In the third (3rd) energy level, there are only three (3) sublevels and these are; s, p and d sublevels.
Silicon is a chemical element that is found in group (4) of the periodic table because it has four (4) electrons in its third and outermost shell.
In its ground state, Silicon (Si) contains the following number of electrons:
Two electrons in its first (n = 1) energy level. Eight electrons in the second (n = 2) energy level.Lastly, it contains four (4) electrons in its third (n = 3) and outer main energy level.Read more: https://brainly.com/question/18214726
A chemist decomposes samples of several compounds; the masses of their constituent elements are listed. Calculate the empirical formula for each compound.
a. 1.245 g Ni, 5.381 g I,
b. 2.677 g Ba, 3.115 g Br,
c. 2.128 g Be, 7.557 g S, 15.107 g
Answers
Answer:
you can see the empirical formula at the pic
The empirical formula for compound (a) is NiI2, (b) is BaBr2 and (c) is BeS.
What is empirical formula?
Empirical formula of a compound is defined as the simplest whole number ratio of atoms present in a compound.
(a) 1.245 g Ni : 5.381 g I
Mole of Ni ; Mole of I = 1.245/59 : 5.381/127 = 0.02 : 0.04 = 1:2
So the formula is NiI2
(b) 2.677 g Ba : 3.115 g Br
Mole of Ba : Mole of Br = 2.677/137 : 3.115/60 = 0.019 : 0.038
= 0.02 : 0.04 = 1:2
So the formula is BaBr2
(c) 2.128 g Be : 7.557 g S
Mole of Be : Mole of S = 2.128/9 : 7.557/32 = 0.2 : 0.2 = 1:1
So the formula is BeS
Thus, empirical formula for compound (a) is NiI2, (b) is BaBr2 and (c) is BeS.
To learn more about empirical formula, refer to the link below:
https://brainly.com/question/11588623
#SPJ2
What is the dependent variable? Be specific. *
If a teacher washes the tops of the student
desks with rubbing alcohol daily, the
spread of germs in the classroom may be
diminished.Be specific when you write your answer HELPPPPP I NEED IT ASAP!
Answers
i need to know how to get a boy to like me
Answers
Answer:
To get a boy to like you you have to 1.dress up cutely
2.talk to him ask him what he likes
3. wear a little bit of make-up not to much
4. wear you hair down and have a little bit of hair on your chest
5. Be really confident and don't wait till a really longg time and mess up like i did.
Explanation:
Answer:
Put your hair down. Also, dont use too much makeup. Just wear mascara and lipgloss. Be kind. Be confident.
Explanation:
If u wear to much makeup he will think ur a try-hard. Stop trying to be cool, it's just dumb. If ur rude, then hes gonna think that u dont like him.
an oxide of copper is decomposed forming copper metal and oxygen gas. a 0.500 g sample of this oxide is decomposed, forming 0.444 g of copper metal. what is the empircal formula of the gold oxide
Answers
Answer:
[tex]Cu_2O[/tex]
Explanation:
Hello.
In this case, since the reaction obeys the law of conservation of mass, since the initial oxide had a mass of 0.500 g and yielded 0.444 g of copper, the mass of oxygen in the oxide is:
[tex]m_O=0.500g-0.444g=0.056g[/tex]
In such a way, we next compute the moles of copper and oxygen by using their atomic masses:
[tex]n_{Cu}=0.444g*\frac{1mol}{63.546 g} =0.00699mol\\\\n_O=0.056g*\frac{1mol}{16.00g}=0.0035mol[/tex]
Next, in order to compute the subscripts of Cu and O on the empirical formula we divide the moles by the fewest moles, in this 0.0035 mol as shown below:
[tex]Cu:\frac{0.00699}{0.0035} =2\\\\O:\frac{0.0035}{0.0035} =1[/tex]
It means that the empirical formula turns out:
[tex]Cu_2O[/tex]
Best regards!
Hydrogen has 3 isotopes. Hydrogen 1, Hydrogen 2 and Hydrogen 3. What is the difference between these 3 is isotopes
Answers
Answer:
Number of neutrons
Explanation:
All have one single proton. Hydrogen has no neutrons. Hydrogen 2 or deuterium has 1 neutron. Hydrogen 3 or tritium has 2 neutrons.
What is the pressure if the height of a column of mercury is 0.20 m and the density of mercury is 13,600 kg/m3? (remember, gravity is 9.81 m/s2)
Answers
Answer:
[tex]p=26683.2Pa[/tex]
Explanation:
Hello,
In this case, since the pressure is computed via:
[tex]p=h*\rho*g[/tex]
Whereas h is the 0.520-m height, [tex]\rho[/tex] is the 13600-kg/m³ density and the g the 9.81-m/s² gravity. Thus, the pressure in Pa is:
[tex]p=0.20m*13,600 \frac{kg}{m^3} *9.81\frac{m}{s^2} \\\\p=26683.2\frac{kg*\frac{m}{s^2} }{m^2} =26683.2\frac{N}{m^2}\\ \\p=26683.2Pa[/tex]
Best regards.
Elements with similar chemical properties are organized in the same
A.) Group
B.) Period
C.) Electron Shell
D.) Row
Answers
Answer:
electron shell is the answer
Determine the volume of 15.5 g of a substance with a density of 6.89 g/ml
Answers
Answer:
The answer is 2.25 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
[tex]volume = \frac{mass}{density} \\ [/tex]
From the question
mass = 15.5 g
density = 6.89 g/mL
We have
[tex]volume = \frac{15.5}{6.89} \\ = 2.249637155...[/tex]
We have the final answer as
2.25 mLHope this helps you
What are the signs that you are getting nervous 18 POINTS )
Answers
- nail biting or perhaps shaking legs
- the feeling of being overwhelmed
- (for some people) the stomach starts to hurt
What is the atomic number of arsenic (As)?
O A. 33
O B. 15
C. 75
D. 4
SUB
Answers
Answer:
33
Explanation: